CASUAL SEMINAR IN BIODIVERSITY AND EVOLUTION BY LAURENT ABI-RACHED & NELS ELDE
On the 19th of February, CIBIO-InBIO will host a casual seminar, by two guest speakers invited by the Immunogenetics, Microbes and Infectious Diseases (IMID) Group.
14h30
DIVERSIFICATION OF THE LEUKOCYTE RECEPTORS FOR MAJOR HISTOCOMPATIBILITY COMPLEX CLASS I IN PRIMATES
Laurent Abi-Rached, Centre National de la Recherche Scientifique (CNRS) - Aix-Marseille Université, Marseille, France
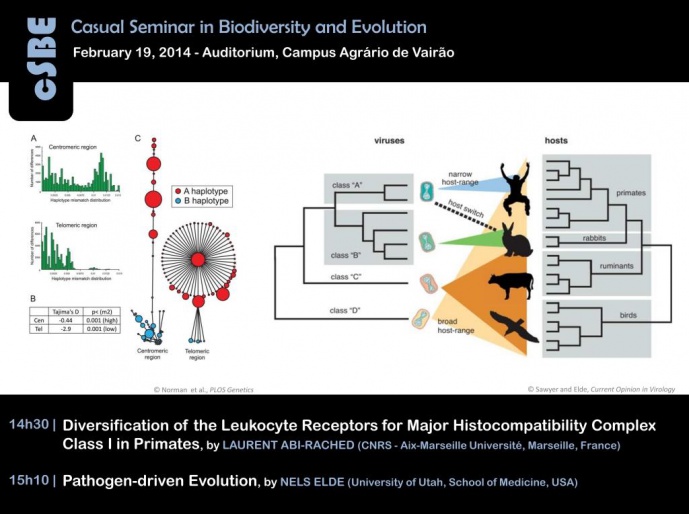
Leukocyte functions in the immune system rely on a battery of cell surface receptors. In higher primates the Killer cell Ig-like Receptor (KIR) and the Leukocyte Ig-Like Receptor (LILR) families represent the variable leukocyte receptors that recognize rapidly-evolving Major Histocompatibility Complex (MHC) class I ligands. The LILR and KIR gene families are both located in the Leukocyte Receptor Complex (LRC), a region on chromosome 19q13 in humans. This presentation will explore past and ongoing efforts to reconstruct the diversification of the KIR and LILR genomic regions in primates, as well as ongoing approaches to study their variability in human populations. Notably, an emphasis will be given on how the evolution of these receptors parallels that of their MHC class I ligands, and on how the rapid evolution of the latter can quickly alter the receptor-ligand interaction, hence creating a major source of immune variability across individuals, populations and species.
15h10
PATHOGEN-DRIVEN EVOLUTION
Nels Elde, University of Utah, School of Medicine, USA
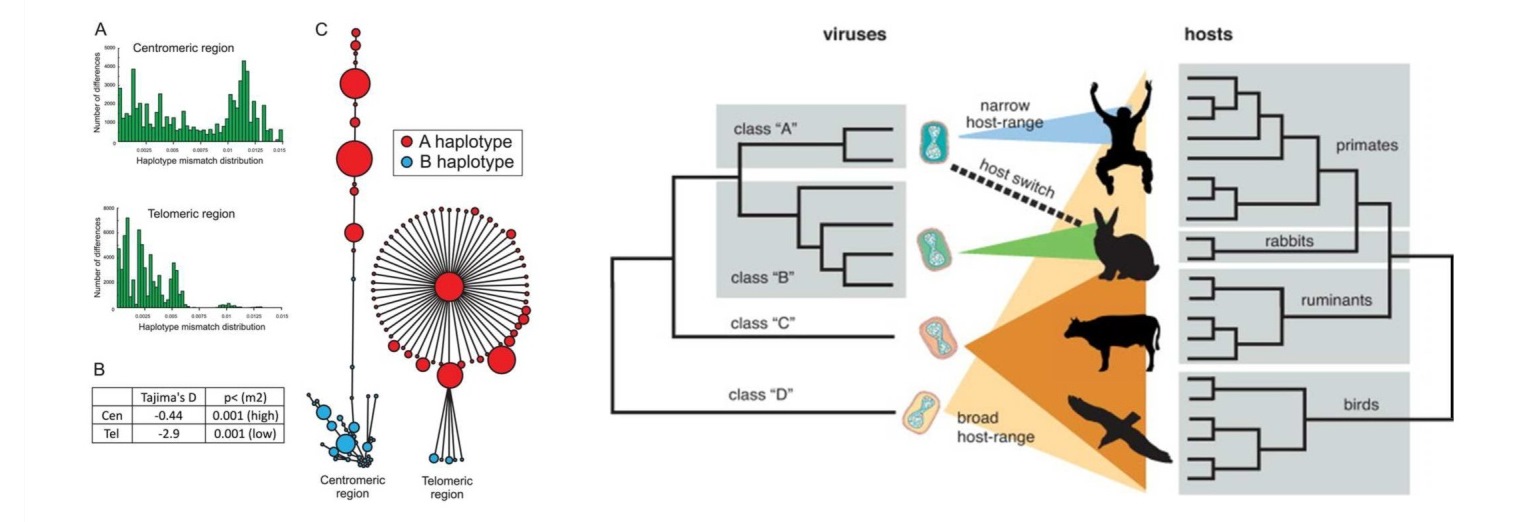
My research program investigates the evolutionary forces shaping genomic and cellular complexity. We focus on the consequences of pathogen-driven evolution on cellular processes and host immunity factors. Protein surfaces at these interfaces often evolve in a manner resembling molecular arms races. We also investigate how pathogens use molecular mimicry to gain advantages against hosts. In addition, we use experimental evolution to determine the evolutionary potential of viruses and understand the rules by which they adapt to overwhelm host defenses.
Host: Pedro Esteves, Immunogenetics, Microbes and Infectious Diseases
Image credits: Norman et al., PLOS Genetics & Sawyer and Elde, Current Opinion in Virology