NATURAL SELECTION RECURRENTLY FAVORED THE EMERGENCE OF MODULAR VARIANTS OF THE BUTYROPHILIN-LIKE 2 RECEPTOR IN HOMINOIDS
CASUAL SEMINAR IN BIODIVERSITY AND EVOLUTION
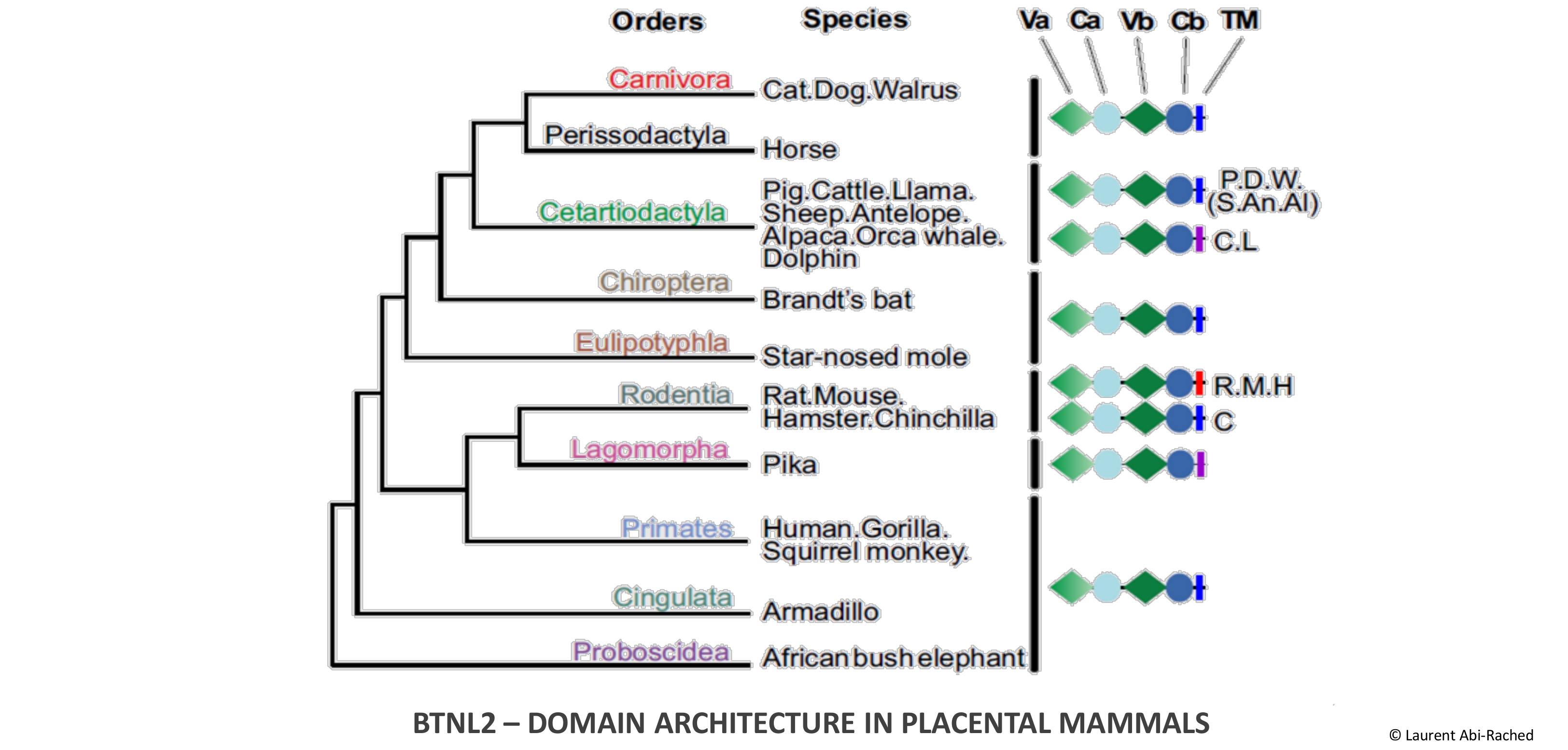
Butyrophilins (BTN) represent a plastic family of immunoregulators that notably includes Butyrophilin-like 2 (BTNL2), an atypical BTN with a unique domain architecture and whose gene is located in the immune gene-rich Human Leukocyte Antigen class II region. BTNL2 contributes to intestinal mucosal immunity in mouse but its function in human remains elusive although genetic data indicate an involvement in autoimmune and inflammatory diseases. To investigate BTNL2 function we studied its evolution in mammals and its diversity in primates and show that while BTNL2 evolution in mammals was marked by positive diversifying selection, its domain structure is conserved across placental mammal orders. Yet, detailed investigation of BTNL2 diversity in hominoids reveals a recurrent formation of truncated variants that occurred independently in all species. This striking parallel evolution creates a balance of complete and truncated alleles in gibbon, orangutan, gorilla and human populations, while in chimpanzee populations the truncated form is now fixed. In humans in particular, careful analysis in a large set of 1,631 individuals representing 26 worldwide populations reveals a highly polymorphic gene whose alleles fall into four ancient lineages encoding distinct structural variants that are maintained at intermediate frequencies by strong balancing selection. We also show that one of these lineages, which only exists in Asia, represents a case of archaic (Denisovan) introgression. Together with data indicating that BTNL2 contains two specialized functional modules, this analysis thus shows that BTNL2 evolution was shaped by strong selective forces and that allelic variation affects both the sequence and the structure of BTNL2.
Laurent Abi-Rached did his PhD work on the emergence of the adaptive immune system in Vertebrates in Marseille in the laboratory of Pierre Pontarotti. In 2002 he then moved to Stanford and the laboratory of Peter Parham to work on the evolution and diversification of the interactions between MHC class I ligands and variable NK cell receptors. A major finding of this research was the discovery of the importance that admixture with archaic humans played for the shaping of modern human immune systems. In 2012, he returned to Marseille to join the IHU Méditerranée Infection and direct a team to investigate how immune variation in populations and in species impacts antimicrobial responses.
[Host: Pedro Esteves, Immunogenetics, Microbes and Infectious Diseases]
Image credits: Laurent Abi-Rached